moving to another question will save this response. what is the product of the following reaction?
Chapter 4: Introduction to How Cells Obtain Energy
4.1 Energy and Metabolism
Learning Objectives
By the end of this section, you will be able to:
- Explain what metabolic pathways are
- State the start and second laws of thermodynamics
- Explain the difference betwixt kinetic and potential energy
- Depict endergonic and exergonic reactions
- Discuss how enzymes function as molecular catalysts
Watch a video about heterotrophs.
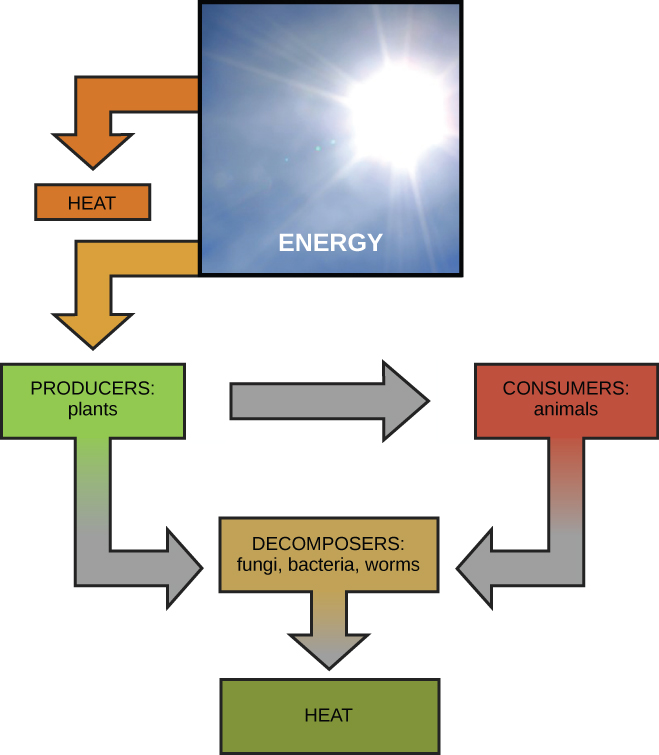
Scientists use the term bioenergetics to draw the concept of energy flow (Figure 4.2) through living systems, such as cells. Cellular processes such every bit the building and breaking downwardly of complex molecules occur through stepwise chemical reactions. Some of these chemic reactions are spontaneous and release energy, whereas others require energy to proceed. Just equally living things must continually consume nutrient to replenish their energy supplies, cells must continually produce more energy to furnish that used by the many energy-requiring chemical reactions that constantly take identify. Together, all of the chemic reactions that have place inside cells, including those that consume or generate energy, are referred to as the jail cell's metabolism.
Metabolic Pathways
Consider the metabolism of saccharide. This is a classic example of one of the many cellular processes that apply and produce free energy. Living things consume sugars equally a major free energy source, because carbohydrate molecules have a peachy deal of energy stored within their bonds. For the near part, photosynthesizing organisms similar plants produce these sugars. During photosynthesis, plants use energy (originally from sunlight) to convert carbon dioxide gas (CO2) into saccharide molecules (like glucose: Chalf-dozenH12O6). They consume carbon dioxide and produce oxygen equally a waste production. This reaction is summarized as:
6CO2 + 6H2O + energy ——-> C6H12O6+ 6O2
Because this procedure involves synthesizing an energy-storing molecule, it requires energy input to continue. During the light reactions of photosynthesis, free energy is provided by a molecule called adenosine triphosphate (ATP), which is the primary free energy currency of all cells. Just as the dollar is used as currency to purchase appurtenances, cells employ molecules of ATP as energy currency to perform immediate work. In contrast, free energy-storage molecules such equally glucose are consumed only to be broken down to use their energy. The reaction that harvests the energy of a sugar molecule in cells requiring oxygen to survive can be summarized by the reverse reaction to photosynthesis. In this reaction, oxygen is consumed and carbon dioxide is released as a waste matter production. The reaction is summarized equally:
C6H12Ohalf dozen + 6O2 ——> 6CO2 + 6HtwoO + free energy
Both of these reactions involve many steps.
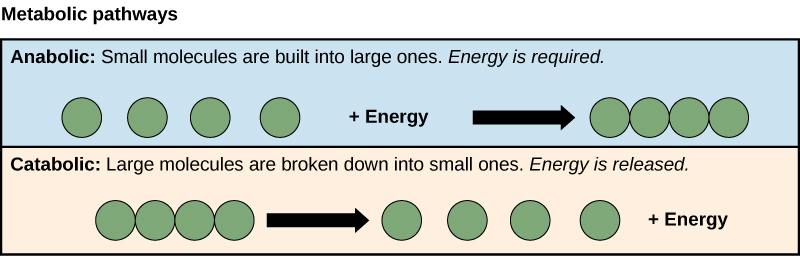
The processes of making and breaking down sugar molecules illustrate ii examples of metabolic pathways. A metabolic pathway is a series of chemical reactions that takes a starting molecule and modifies it, step-by-step, through a series of metabolic intermediates, somewhen yielding a final product. In the case of sugar metabolism, the commencement metabolic pathway synthesized saccharide from smaller molecules, and the other pathway broke sugar downwards into smaller molecules. These 2 contrary processes—the first requiring energy and the second producing energy—are referred to every bit anabolic pathways (building polymers) and catabolic pathways (breaking downwardly polymers into their monomers), respectively. Consequently, metabolism is composed of synthesis (anabolism) and degradation (catabolism) (Figure four.iii).
It is important to know that the chemical reactions of metabolic pathways practise not accept place on their own. Each reaction pace is facilitated, or catalyzed, by a poly peptide called an enzyme. Enzymes are important for catalyzing all types of biological reactions—those that crave energy as well every bit those that release energy.
Energy
Thermodynamics refers to the report of energy and energy transfer involving physical matter. The matter relevant to a particular case of energy transfer is chosen a system, and everything exterior of that matter is chosen the environs. For instance, when heating a pot of h2o on the stove, the system includes the stove, the pot, and the water. Free energy is transferred inside the system (between the stove, pot, and h2o). There are 2 types of systems: open and airtight. In an open system, free energy can be exchanged with its surroundings. The stovetop system is open because heat can be lost to the air. A closed organisation cannot exchange energy with its environs.
Biological organisms are open systems. Energy is exchanged betwixt them and their environs as they use energy from the sunday to perform photosynthesis or consume energy-storing molecules and release energy to the environment by doing piece of work and releasing heat. Similar all things in the physical world, free energy is subject to physical laws. The laws of thermodynamics govern the transfer of free energy in and among all systems in the universe.
In general, free energy is defined equally the ability to do work, or to create some kind of change. Energy exists in different forms. For example, electrical energy, light energy, and heat free energy are all different types of free energy. To appreciate the way energy flows into and out of biological systems, it is important to understand two of the physical laws that govern energy.
Thermodynamics
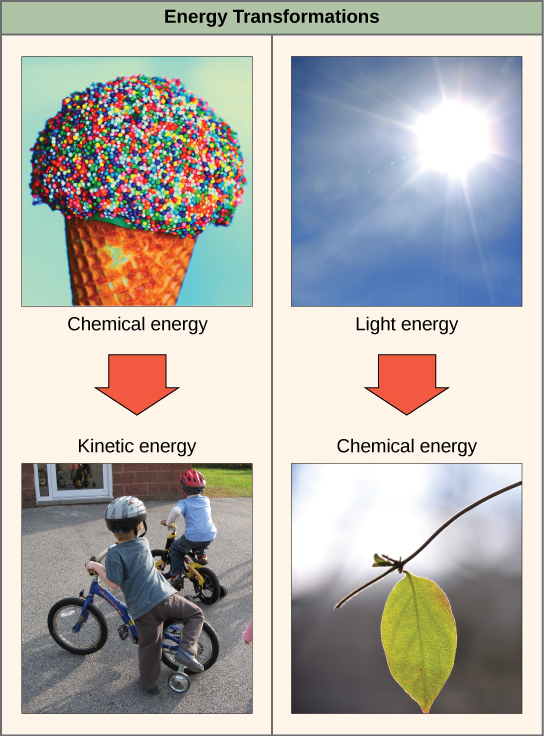
The first law of thermodynamics states that the total amount of energy in the universe is constant and conserved. In other words, in that location has e'er been, and always volition be, exactly the aforementioned amount of free energy in the universe. Free energy exists in many different forms. According to the first law of thermodynamics, free energy may be transferred from place to place or transformed into different forms, but it cannot be created or destroyed. The transfers and transformations of energy take place around u.s.a. all the time. Low-cal bulbs transform electrical energy into light and heat energy. Gas stoves transform chemical energy from natural gas into heat free energy. Plants perform one of the nigh biologically useful energy transformations on world: that of converting the energy of sunlight to chemical energy stored within organic molecules (Figure 4.2). Some examples of free energy transformations are shown in Figure 4.4.
The challenge for all living organisms is to obtain energy from their environs in forms that they tin transfer or transform into usable free energy to practice piece of work. Living cells accept evolved to meet this challenge. Chemical energy stored within organic molecules such as sugars and fats is transferred and transformed through a series of cellular chemical reactions into energy within molecules of ATP. Free energy in ATP molecules is easily accessible to do piece of work. Examples of the types of work that cells need to practice include edifice complex molecules, transporting materials, powering the motion of cilia or flagella, and contracting muscle fibers to create motility.
A living prison cell's primary tasks of obtaining, transforming, and using energy to do work may seem simple. However, the second police of thermodynamics explains why these tasks are harder than they appear. All energy transfers and transformations are never completely efficient. In every free energy transfer, some amount of energy is lost in a form that is unusable. In most cases, this form is heat energy. Thermodynamically, heat free energy is divers every bit the free energy transferred from one system to another that is not work. For example, when a light bulb is turned on, some of the free energy beingness converted from electric energy into light energy is lost as rut free energy. Likewise, some energy is lost as heat energy during cellular metabolic reactions.
An important concept in physical systems is that of social club and disorder. The more than energy that is lost by a system to its surround, the less ordered and more random the organization is. Scientists refer to the measure of randomness or disorder within a system as entropy. Loftier entropy means high disorder and depression energy. Molecules and chemical reactions have varying entropy as well. For example, entropy increases as molecules at a high concentration in one identify diffuse and spread out. The second law of thermodynamics says that free energy will always be lost every bit heat in free energy transfers or transformations.
Living things are highly ordered, requiring constant free energy input to be maintained in a state of low entropy.
Potential and Kinetic Free energy
When an object is in motion, at that place is energy associated with that object. Think of a wrecking ball. Even a slow-moving wrecking brawl can do a great deal of damage to other objects. Energy associated with objects in motion is chosen kinetic free energy (Figure 4.v). A speeding bullet, a walking person, and the rapid movement of molecules in the air (which produces heat) all have kinetic energy.
Now what if that aforementioned motionless wrecking brawl is lifted two stories above ground with a crane? If the suspended wrecking ball is unmoving, is there free energy associated with it? The respond is yep. The free energy that was required to lift the wrecking ball did not disappear, but is now stored in the wrecking brawl by virtue of its position and the force of gravity acting on information technology. This blazon of energy is called potential energy (Effigy 4.5). If the ball were to fall, the potential energy would exist transformed into kinetic free energy until all of the potential energy was exhausted when the ball rested on the ground. Wrecking balls also swing like a pendulum; through the swing, in that location is a abiding change of potential energy (highest at the pinnacle of the swing) to kinetic energy (highest at the bottom of the swing). Other examples of potential free energy include the energy of water held behind a dam or a person about to skydive out of an airplane.

Potential energy is not only associated with the location of matter, but also with the structure of matter. Even a bound on the basis has potential free energy if information technology is compressed; so does a rubber band that is pulled taut. On a molecular level, the bonds that agree the atoms of molecules together exist in a particular structure that has potential energy. Call back that anabolic cellular pathways crave free energy to synthesize complex molecules from simpler ones and catabolic pathways release energy when complex molecules are broken downwards. The fact that energy can be released by the breakup of certain chemical bonds implies that those bonds have potential energy. In fact, at that place is potential energy stored within the bonds of all the nutrient molecules we swallow, which is eventually harnessed for employ. This is because these bonds can release energy when broken. The blazon of potential energy that exists within chemical bonds, and is released when those bonds are broken, is chosen chemical free energy. Chemical energy is responsible for providing living cells with energy from food. The release of energy occurs when the molecular bonds within food molecules are broken.
Picket a video about kilocalories.
Concept in Action

Visit the site and select "Pendulum" from the "Work and Energy" menu to see the shifting kinetic and potential energy of a pendulum in motion.
Gratuitous and Activation Energy
After learning that chemical reactions release energy when energy-storing bonds are broken, an important next question is the following: How is the energy associated with these chemical reactions quantified and expressed? How can the energy released from one reaction be compared to that of another reaction? A measurement of gratis energy is used to quantify these free energy transfers. Recall that co-ordinate to the second law of thermodynamics, all energy transfers involve the loss of some amount of free energy in an unusable form such as heat. Free energy specifically refers to the energy associated with a chemical reaction that is available after the losses are deemed for. In other words, gratuitous energy is usable energy, or energy that is available to do work.
If energy is released during a chemic reaction, then the change in free energy, signified as ∆Thousand (delta G) will exist a negative number. A negative change in free energy also means that the products of the reaction have less energy than the reactants, because they release some free energy during the reaction. Reactions that take a negative modify in free energy and consequently release free energy are chosen exergonic reactions. Call up: exergonic ways free energy is exiting the organisation. These reactions are too referred to as spontaneous reactions, and their products have less stored energy than the reactants. An important distinction must exist drawn between the term spontaneous and the idea of a chemic reaction occurring immediately. Contrary to the everyday apply of the term, a spontaneous reaction is non one that all of a sudden or quickly occurs. The rusting of iron is an instance of a spontaneous reaction that occurs slowly, little by petty, over time.
If a chemical reaction absorbs energy rather than releases energy on balance, then the ∆1000 for that reaction will be a positive value. In this instance, the products have more free energy than the reactants. Thus, the products of these reactions tin be idea of as energy-storing molecules. These chemical reactions are chosen endergonic reactions and they are not-spontaneous. An endergonic reaction will non accept identify on its ain without the improver of gratis energy.

Wait at each of the processes shown and determine if it is endergonic or exergonic.
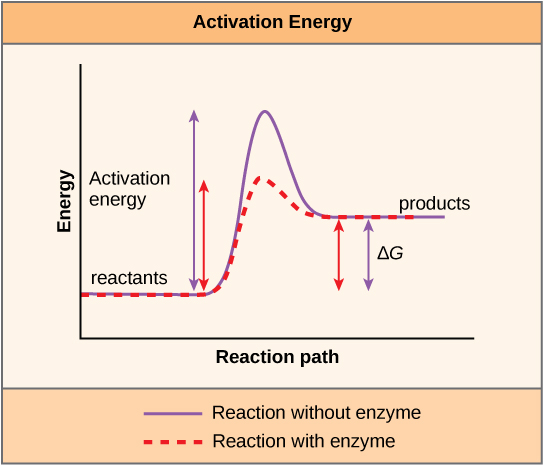
There is another important concept that must be considered regarding endergonic and exergonic reactions. Exergonic reactions crave a small amount of energy input to get going, before they can proceed with their energy-releasing steps. These reactions have a net release of energy, but even so crave some energy input in the starting time. This minor amount of energy input necessary for all chemic reactions to occur is called the activation free energy.
Concept in Activeness

Watch an animation of the move from energy to transition land of the reaction.
Enzymes
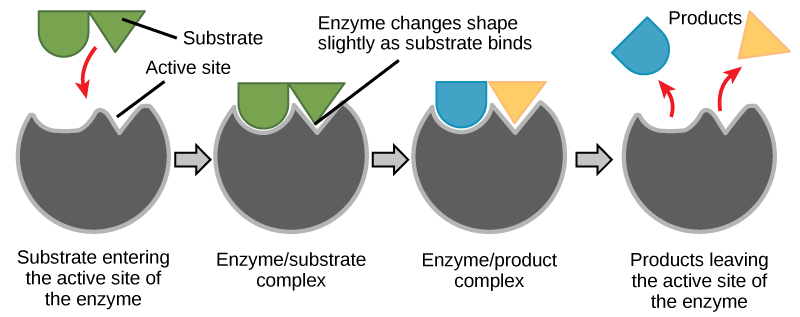
A substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. Nigh enzymes are proteins and perform the critical task of lowering the activation energies of chemical reactions inside the cell. Most of the reactions critical to a living prison cell happen also slowly at normal temperatures to be of any use to the jail cell. Without enzymes to speed up these reactions, life could not persist. Enzymes practice this by binding to the reactant molecules and property them in such a way as to make the chemical bond-breaking and -forming processes take place more hands. It is important to call up that enzymes do not change whether a reaction is exergonic (spontaneous) or endergonic. This is because they exercise not alter the free free energy of the reactants or products. They only reduce the activation energy required for the reaction to go forward (Figure 4.7). In add-on, an enzyme itself is unchanged by the reaction information technology catalyzes. Once one reaction has been catalyzed, the enzyme is able to participate in other reactions.
The chemic reactants to which an enzyme binds are called the enzyme'southward substrates. There may be one or more substrates, depending on the item chemic reaction. In some reactions, a single reactant substrate is cleaved down into multiple products. In others, two substrates may come up together to create i larger molecule. Two reactants might also enter a reaction and both go modified, but they exit the reaction every bit two products. The location within the enzyme where the substrate binds is called the enzyme's active site. The active site is where the "action" happens. Since enzymes are proteins, at that place is a unique combination of amino acid side chains inside the active site. Each side chain is characterized past unlike backdrop. They can be big or small, weakly acidic or basic, hydrophilic or hydrophobic, positively or negatively charged, or neutral. The unique combination of side bondage creates a very specific chemical environs inside the agile site. This specific surroundings is suited to bind to ane specific chemic substrate (or substrates).
Agile sites are field of study to influences of the local environment. Increasing the environmental temperature generally increases reaction rates, enzyme-catalyzed or otherwise. However, temperatures exterior of an optimal range reduce the charge per unit at which an enzyme catalyzes a reaction. Hot temperatures volition eventually crusade enzymes to denature, an irreversible change in the three-dimensional shape and therefore the function of the enzyme. Enzymes are also suited to function all-time inside a certain pH and salt concentration range, and, as with temperature, extreme pH, and table salt concentrations tin cause enzymes to denature.
For many years, scientists thought that enzyme-substrate bounden took place in a uncomplicated "lock and key" fashion. This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step. Still, electric current research supports a model called induced fit (Figure four.8). The induced-fit model expands on the lock-and-key model by describing a more dynamic binding between enzyme and substrate. Every bit the enzyme and substrate come together, their interaction causes a mild shift in the enzyme's structure that forms an ideal binding system betwixt enzyme and substrate.
Concept in Action

View an animation of induced fit.
When an enzyme binds its substrate, an enzyme-substrate complex is formed. This circuitous lowers the activation free energy of the reaction and promotes its rapid progression in one of multiple possible means. On a basic level, enzymes promote chemical reactions that involve more than 1 substrate by bringing the substrates together in an optimal orientation for reaction. Another way in which enzymes promote the reaction of their substrates is by creating an optimal environment within the active site for the reaction to occur. The chemic properties that emerge from the particular organisation of amino acid R groups within an active site create the perfect environment for an enzyme's specific substrates to react.
The enzyme-substrate complex can besides lower activation energy by compromising the bond construction so that it is easier to break. Finally, enzymes can also lower activation energies by taking part in the chemical reaction itself. In these cases, information technology is of import to remember that the enzyme will always render to its original state by the completion of the reaction. One of the hallmark backdrop of enzymes is that they remain ultimately unchanged by the reactions they catalyze. After an enzyme has catalyzed a reaction, it releases its product(s) and can catalyze a new reaction.
It would seem ideal to take a scenario in which all of an organism's enzymes existed in abundant supply and functioned optimally nether all cellular weather condition, in all cells, at all times. Even so, a diversity of mechanisms ensures that this does not happen. Cellular needs and conditions constantly vary from cell to prison cell, and modify inside private cells over time. The required enzymes of breadbasket cells differ from those of fat storage cells, skin cells, claret cells, and nerve cells. Furthermore, a digestive organ prison cell works much harder to process and break down nutrients during the time that closely follows a repast compared with many hours later a meal. As these cellular demands and atmospheric condition vary, so must the amounts and functionality of unlike enzymes.
Since the rates of biochemical reactions are controlled by activation energy, and enzymes lower and decide activation energies for chemic reactions, the relative amounts and operation of the variety of enzymes within a cell ultimately determine which reactions will proceed and at what rates. This determination is tightly controlled in cells. In certain cellular environments, enzyme activity is partly controlled by environmental factors like pH, temperature, salt concentration, and, in some cases, cofactors or coenzymes.
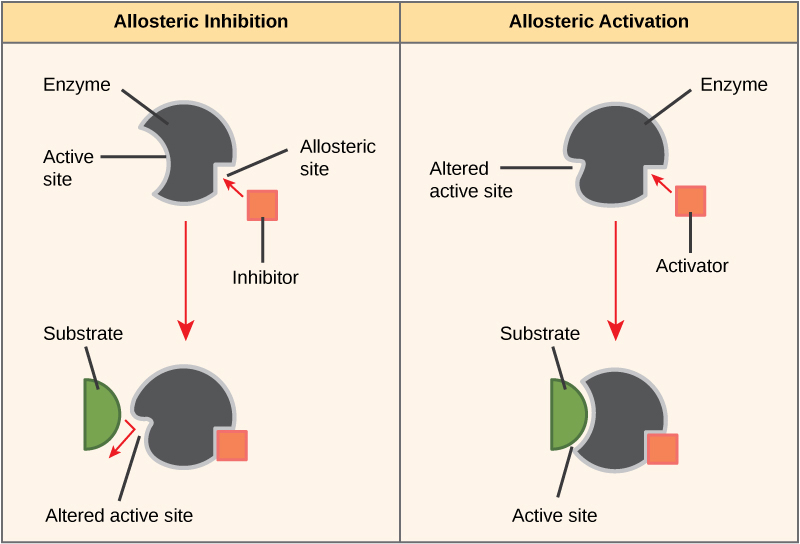
Enzymes tin can also be regulated in ways that either promote or reduce enzyme action. There are many kinds of molecules that inhibit or promote enzyme function, and various mechanisms past which they do so. In some cases of enzyme inhibition, an inhibitor molecule is similar enough to a substrate that it tin bind to the active site and simply block the substrate from binding. When this happens, the enzyme is inhibited through competitive inhibition, because an inhibitor molecule competes with the substrate for bounden to the active site.
On the other hand, in noncompetitive inhibition, an inhibitor molecule binds to the enzyme in a location other than the active site, called an allosteric site, but still manages to block substrate binding to the agile site. Some inhibitor molecules bind to enzymes in a location where their binding induces a conformational alter that reduces the analogousness of the enzyme for its substrate. This type of inhibition is called allosteric inhibition (Figure four.9). Well-nigh allosterically regulated enzymes are made upwardly of more than one polypeptide, meaning that they have more than one protein subunit. When an allosteric inhibitor binds to a region on an enzyme, all active sites on the protein subunits are changed slightly such that they demark their substrates with less efficiency. There are allosteric activators as well as inhibitors. Allosteric activators bind to locations on an enzyme away from the agile site, inducing a conformational modify that increases the analogousness of the enzyme'south active site(south) for its substrate(south) (Figure iv.9).
Through the Indigenous Lens
Plants cannot run or hide from their predators and have evolved many strategies to deter those who would swallow them. Think of thorns, irritants and secondary metabolites: these are compounds that practise not directly help the plant grow, simply are made specifically to continue predators away. Secondary metabolites are the most mutual manner plants deter predators. Some examples of secondary metabolites are atropine, nicotine, THC and caffeine. Humans have establish these secondary metabolite compounds a rich source of materials for medicines. It is estimated that 90% of the drugs in the modern chemist's have their "roots" in these secondary metabolites.
First peoples herbal treatments revealed these secondary metabolites to the world. For case, Ethnic peoples have long used the bark of willow shrubs and alder trees for a tea, tonic or poultice to reduce inflammation. You will larn more nigh the inflammation response by the allowed arrangement in chapter 11.

Both willow and alder bark comprise the compound salicin. Virtually of us accept this chemical compound in our medicine closet in the course of salicylic acid or aspirin. Aspirin has been proved to reduce pain and inflammation, and once in our cells salicin converts to salicylic acid.
Then how does it piece of work? Salicin or aspirin acts as an enzyme inhibitor. In the inflammatory response 2 enzymes, COX1 and COX2 are key to this procedure. Salicin or aspirin specifically modifies an amino acid (serine) in the active site of these 2 related enzymes. This modification of the active sites does not allow the normal substrate to bind and so the inflammatory process is disrupted. As you have read in this chapter, this makes information technology competitive enzyme inhibitor.
Pharmaceutical Drug Developer

Enzymes are cardinal components of metabolic pathways. Understanding how enzymes work and how they can be regulated are cardinal principles behind the evolution of many of the pharmaceutical drugs on the market place today. Biologists working in this field collaborate with other scientists to design drugs (Figure 4.11).
Consider statins for case—statins is the name given to i form of drugs that tin can reduce cholesterol levels. These compounds are inhibitors of the enzyme HMG-CoA reductase, which is the enzyme that synthesizes cholesterol from lipids in the body. By inhibiting this enzyme, the level of cholesterol synthesized in the body can exist reduced. Similarly, acetaminophen, popularly marketed under the brand proper noun Tylenol, is an inhibitor of the enzyme cyclooxygenase. While information technology is used to provide relief from fever and inflammation (pain), its mechanism of activity is even so not completely understood.
How are drugs discovered? I of the biggest challenges in drug discovery is identifying a drug target. A drug target is a molecule that is literally the target of the drug. In the instance of statins, HMG-CoA reductase is the drug target. Drug targets are identified through painstaking research in the laboratory. Identifying the target alone is non enough; scientists besides demand to know how the target acts inside the cell and which reactions become awry in the case of disease. Once the target and the pathway are identified, and so the actual process of drug design begins. In this stage, chemists and biologists work together to design and synthesize molecules that can block or activate a particular reaction. However, this is only the beginning: If and when a drug prototype is successful in performing its function, then it is subjected to many tests from in vitro experiments to clinical trials before it can get approving from the U.Southward. Food and Drug Assistants to be on the market.
Many enzymes practise not work optimally, or fifty-fifty at all, unless spring to other specific non-protein helper molecules. They may bond either temporarily through ionic or hydrogen bonds, or permanently through stronger covalent bonds. Bounden to these molecules promotes optimal shape and function of their respective enzymes. Two examples of these types of helper molecules are cofactors and coenzymes. Cofactors are inorganic ions such as ions of iron and magnesium. Coenzymes are organic helper molecules, those with a bones diminutive structure made upwardly of carbon and hydrogen. Like enzymes, these molecules participate in reactions without being inverse themselves and are ultimately recycled and reused. Vitamins are the source of coenzymes. Some vitamins are the precursors of coenzymes and others deed directly as coenzymes. Vitamin C is a direct coenzyme for multiple enzymes that take part in building the important connective tissue, collagen. Therefore, enzyme part is, in part, regulated past the abundance of various cofactors and coenzymes, which may be supplied past an organism's nutrition or, in some cases, produced by the organism.
Feedback Inhibition in Metabolic Pathways
Molecules tin regulate enzyme function in many means. The major question remains, however: What are these molecules and where exercise they come from? Some are cofactors and coenzymes, equally you have learned. What other molecules in the cell provide enzymatic regulation such as allosteric modulation, and competitive and non-competitive inhibition? Perhaps the most relevant sources of regulatory molecules, with respect to enzymatic cellular metabolism, are the products of the cellular metabolic reactions themselves. In a most efficient and elegant way, cells have evolved to utilise the products of their own reactions for feedback inhibition of enzyme activity. Feedback inhibition involves the use of a reaction product to regulate its own farther production (Figure 4.12). The cell responds to an affluence of the products by slowing downwards production during anabolic or catabolic reactions. Such reaction products may inhibit the enzymes that catalyzed their product through the mechanisms described above.
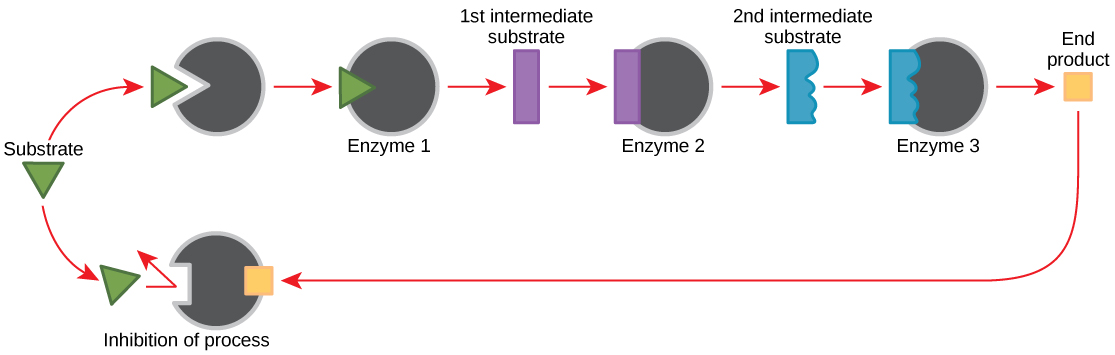
The production of both amino acids and nucleotides is controlled through feedback inhibition. Additionally, ATP is an allosteric regulator of some of the enzymes involved in the catabolic breakup of saccharide, the process that creates ATP. In this manner, when ATP is in abundant supply, the jail cell tin can prevent the production of ATP. On the other manus, ADP serves as a positive allosteric regulator (an allosteric activator) for some of the same enzymes that are inhibited past ATP. Thus, when relative levels of ADP are loftier compared to ATP, the cell is triggered to produce more ATP through sugar catabolism.
Section Summary
Cells perform the functions of life through various chemic reactions. A cell's metabolism refers to the combination of chemic reactions that take place within it. Catabolic reactions break down complex chemicals into simpler ones and are associated with energy release. Anabolic processes build complex molecules out of simpler ones and require energy.
In studying energy, the term organisation refers to the matter and surroundings involved in energy transfers. Entropy is a mensurate of the disorder of a organisation. The concrete laws that depict the transfer of energy are the laws of thermodynamics. The first law states that the full corporeality of energy in the universe is constant. The 2nd police of thermodynamics states that every energy transfer involves some loss of free energy in an unusable form, such as oestrus free energy. Free energy comes in dissimilar forms: kinetic, potential, and free. The modify in gratuitous energy of a reaction tin exist negative (releases free energy, exergonic) or positive (consumes free energy, endergonic). All reactions require an initial input of free energy to go along, called the activation free energy.
Enzymes are chemical catalysts that speed upwards chemical reactions by lowering their activation energy. Enzymes take an agile site with a unique chemic surroundings that fits particular chemical reactants for that enzyme, called substrates. Enzymes and substrates are idea to bind according to an induced-fit model. Enzyme activeness is regulated to conserve resources and respond optimally to the environment.
Glossary
activation energy: the amount of initial energy necessary for reactions to occur
active site: a specific region on the enzyme where the substrate binds
allosteric inhibition: the mechanism for inhibiting enzyme activity in which a regulatory molecule binds to a 2nd site (not the agile site) and initiates a conformation modify in the active site, preventing binding with the substrate
anabolic: describes the pathway that requires a net energy input to synthesize complex molecules from simpler ones
bioenergetics: the concept of energy menstruation through living systems
catabolic: describes the pathway in which circuitous molecules are broken down into simpler ones, yielding energy equally an additional production of the reaction
competitive inhibition: a general mechanism of enzyme activity regulation in which a molecule other than the enzyme'due south substrate is able to bind the active site and prevent the substrate itself from binding, thus inhibiting the overall charge per unit of reaction for the enzyme
endergonic: describes a chemical reaction that results in products that shop more than chemical potential energy than the reactants
enzyme: a molecule that catalyzes a biochemical reaction
exergonic: describes a chemical reaction that results in products with less chemical potential energy than the reactants, plus the release of gratuitous free energy
feedback inhibition: a mechanism of enzyme activeness regulation in which the product of a reaction or the final product of a series of sequential reactions inhibits an enzyme for an earlier footstep in the reaction series
heat free energy: the free energy transferred from ane organisation to another that is not work
kinetic free energy: the type of energy associated with objects in motion
metabolism: all the chemical reactions that take place inside cells, including those that utilise energy and those that release energy
noncompetitive inhibition: a full general mechanism of enzyme activity regulation in which a regulatory molecule binds to a site other than the active site and prevents the active site from binding the substrate; thus, the inhibitor molecule does not compete with the substrate for the agile site; allosteric inhibition is a form of noncompetitive inhibition
potential energy: the type of energy that refers to the potential to exercise piece of work
substrate: a molecule on which the enzyme acts
thermodynamics: the science of the relationships betwixt heat, energy, and work
Source: https://opentextbc.ca/biology/chapter/4-1-energy-and-metabolism/
0 Response to "moving to another question will save this response. what is the product of the following reaction?"
Post a Comment